What is “Kanemi Yusho”?
“Kanemi Yusho” was caused by the contamination of heated Polychlororinated Bisphenyls (PCBs) for deodorization in the manufacturing process of rice bran oil at the Kanemi Warehouse Co. Ltd., (Kokura-ward, Kitakyushu, Fukuoka, Japan) in 1968.People who ingested contaminated rice bran oil developed skin conditions, such as chloracne, which is characterized by black and white pustules, as well as liver and kidney diseases. A total of 15,000 peoples were affected. The sale of the contaminated rice bran oil was immediately banned, and it was concluded that the causative agent of “Kanemi Yusho” was Polychlorinated Dibenzofurans (PCDFs) derived from heated PCBs [1]. There toxicity are expressed after the activation by Aryl Hydrocarbon Receptor (AhR) [2].
The Transgenerational Inheritance Problem of “Kanemi Yusho”
Research has been primarily conducted by the Department of Dermatology, Faculty of Medicine, Kyushu University, on the treatment of “Kanemi Yusho” and its causative agent. The Ministry of Health and Welfare, Japan also established “The Kanemi Yusho Treatment and Research Group in Japan” which continued an extensive research on the diagnosis, treatment, and detoxification on patients of “Kameni Yusho” [1]. In recent years, however, an extremely serious problem of inter/trans generational transmission of “Kanemi Yusho” has arisen in the next generation and beyond, in which similar symptoms were experienced. The research group conducted a survey on the subsequent generations of “Kanemi Yusho” from 2021 on the volunteer patients and an interim report was published in June, 2022 [2]. The number of patients surveyed after the next generations are approximately 400, including 66 third generation patients. According to the patients’ chief complaints, the same symptoms as those reported in the originally affected patients were observed at a relatively high frequency. This cannot be explained solely by the transplacental or breast milk toxicity, which is different from the direct effects of PCDFs must be considered. A conceptual diagram of this process is shown in fFigure 1
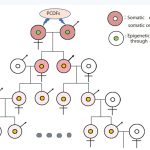
Figure 1 Conceptual map of the pedigree in “Kanemi Yusho”
Table 1
EDCs : species: reference/>
Vinclozolin : Rats : Anway et al.(2005) (6)/>
Methoxychlor (MXC): Rats : Anway et al.(2005) (6)/>
2,3,7,8-Tetrachlorobibenzi-p-dioxin(TCDD): Rats: Mannikkam et al. (2012) />
Dichlorodiphenyltrichloroethane (DDT): Rats : Skinner et al. (2013) />
Hydrocarbon(JP-8) : Rats : Tracey et al. (2013) />
Bisphenol (BPA) Rats : Li et al. (2014) />
P’-DDE : Mouse : Song et al.(2014) />
Phthalates Rats : Mannikkam et al. (2014) />
Atrazine : Mouse : Hao et al. (2016) />
Benzo(a)Pyrene: Mouse : Brevik et al. (2016)/>
Glyphosate (Roundup): Rats : Kusbad et al. (2020) />
Polychlorinated dibenzofurans (PCDFs): unknown
Transgenerational Epigenetic Inheritance
Recently, the term “epigenetics” has become increasingly spread in the fi eld of medicine and biology. Epigenetics is a complex biological phenomenon, involving DNA methylation and various surrounding histone modifications, as well as various non-coding RNAs, and is involved in regulation on gene expression. Epigenetics plays a major role in cell differentiation during development from fertilized eggs and in the maintenance of adult life. The term “environmental epigenetics” refers to the disruption of epigenetics by various environmental factors. Somatic and germ cells are affected separately by environmental epigenetics [4,5]. Somatic cells are affected in the regulation of epigenetics by various environmental factors in various pathways, but this effect exert only in the relevant generation. Conversely, for germ cells, studies have been mainly conducted by Skinner’s group (Washington State University) since 2005 using Vinclozolin and Methoxychlor, they possess the potency to induce Transgenerational Epigenetic Inheritance (TEI) in rodent experiments [6]. From the reports of Skinner’s group, many other researchers have shown that most of Endocrine Disrupting Chemicals (EDCs) possess the capacity to induce TEI [7,8]. The results of TEI in various EDCs are summarized in Table 1. TEI is a phenomenon in which a generation is exposed to an environmental factor, and after successive mating with untreated individuals, toxic events owing to epigenetic changes in germ cells are observed even in the unexposed generations [4,5]. Therefore, the causative agent of “Kanemi Yusho”, PCDFs needs to be reconsidered from this point of view. If the transgenerational effects of “Kanemi Yusho” are elucidated the TEI can provide detailed data on the TEI in humans caused by a specific chemical substance. These results will contribute to developing of treatment methods and improving of welfare of patients of “Kanemi Yusho”. It is noteworthy to add that TEIs in humans, unlike genetic disease, are unlikely to be passed on indefinitely.
To investigate whether the transgenerational problem of “Kanemi Yusho” can be attributed to TEI, it is necessary to examine blood and other cell samples from patients and their off spring in families with known maternal and paternal intake. And an important genomic region associated with TEI may be the imprinted gene, IGF2/H19. It is well known that the relationship between EDCs, AhR, and the imprinted gene IGF2/H19 has been much discussed [9,10]. It is clarified that TEI induced by various EDCs might be originated from the activations of AhR and it disturbed the insulin-like growth factor system [8,9]. We are planning to analyze on the familiar incidence of “Kanemi Yusho” patients from this point of view in near future. And unite various epigenetic phenomena, such as DNA methylation, modification of histones, and the contribution of non-coding RNA to understanding epigenomic system by EDCs.
The Impact of Endocrine Disrupting Chemicals (Environmental Hormones) on Future Generations
As previously mentioned, animal studies have revealed that TEIs occur by endocrine disrupting chemicals (ECDs). EDCs which possess TEI action are listed in Table 1. The general public and the surrounding environments have already been contaminated by these EDCs, even in low concentrations. And these effects might add up years and years. The possibility that TEIs may harm future generations of the general public cannot be ignored. Toxicological scientists must estimate the risk of EDCs in future generations using appropriate statistical assessment models.
References
1. Kuratsune M, Yoshimura H, Hori Y, Okamura M, Masuda Y (edis), Yusho: A Human Disaster by PCBs and related compounds. How to cite this article: Shibuya T, Horiya Y. Kanemi Yusho and Transgenerational Epigenetic Inheritance. 2023 Mar 30; 4(3): 543-545. doi: 10.37871/jbres1708, Article ID: JBRES1708, Available at: https://www.jelsciences.com/articles/jbres1708.pdf Kyushu University Press. 1996.
2. Furue M, Ishii Y, Tsukimori K, Tsuji G. Aryl Hydrocarbon Receptor and Dioxin-Related Health Hazards-Lessons from Yusho. Int J Mol Sci. 2021 Jan 12;22(2):708. doi: 10.3390/ijms22020708. PMID: 33445793; PMCID: PMC7828254.
3. Study group for treatment of Yusho in Japan (Leader Dr. G. Tsuji, Faculty of Medicine, Kyushu University: An interim report on the generational effects of Kanemi Yusho (in Japanese). 2022.
4. Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011 Jul;6(7):838-42. doi: 10.4161/epi.6.7.16537. Epub 2011 Jul 1. PMID: 21637037; PMCID: PMC5703187.
5. Skinner MK. Environmental stress and epigenetic transgenerational inheritance. BMC Medicine. 2014;12:153-157.
6. Anway MD, Cupp AS, Uzumci M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466-1469.
7. van Cauwenbergh O, Di Serafi no A, Tygat J, Soubry A. Transgenerational epigenetic effects from male exposure to endocrine-disrupting compounds: A systematic review on research in mammals. Clinic Epigenetics. 2020;12:65-87.
8. Shibuya T, Horiya Y. Endocrine disrupting chemicals (EDCs) induce transgenerational epigenetic inheritance (TEI). Proceeding of the 49th Soc. Japanese Toxicology, Sapporo, Japan. 2022.
9. Talia C, Connolly L, Fowler PA. The insulin-like growth factor system: A target for endocrine disruptors? Environ Int. 2021 Feb;147:106311. doi: 10.1016/j.envint.2020.106311. Epub 2020 Dec 18. PMID: 33348104.
10. Gaspari L, Paris F, Kalfa N, Soyer-Gobillard MO, Sultan C, Hamamah S. Experimental Evidence of 2,3,7,8-Tetrachlordibenzo-p-Dioxin (TCDD) Transgenerational Effects on Reproductive Health. Int J Mol Sci. 2021 Aug 23;22(16):9091. doi: 10.3390/ijms22169091. PMID: 34445797; PMCID: PMC8396488